Abstract
Introduction The Nordic NLG-T-01 was a large phase II trial evaluating the efficacy of bi-weekly etoposide-containing chemotherapy consolidated by autologous transplant in newly diagnosed patients with systemic peripheral T-cell lymphomas (PTCL) (d'Amore et al, JCO 2012). The final analysis of the trial showed female sex and anaplastic large cell (ALCL) histology to be predictive of a favourable outcome. Here, we present a long-term follow-up evaluating the outcome of NLG-T-01 patients according to clinico-pathological parameters and biological features, in particular rearrangements of the dual specificity phosphatase 22 (DUSP22-IRF4) and the TP63 gene.
Methods A cohort of 160 adult PTCL patients aged 18 to 67 years were enrolled. Anaplastic lymphoma kinase (alk)-positive anaplastic large-cell lymphoma (ALCL), primary cutaneous and primary leukemic PTCL were excluded. Considering the high median age of the study population at the time of enrolment (57 yrs) and the length of follow-up, we analyzed, in addition to overall (OS) and progression-free survival (PFS), also disease-specific survival (DSS). Time-to-event end points were calculated by means of Kaplan-Meier estimates. Log-rank tests and Cox regression models were used to analyze prognostic factors. A trial-specific tissue micro-array (TMA) was constructed with those pre-therapeutic biopsies, where sufficient tissue was still available for analysis (n=87). Fluorescent in situ hybridization (FISH) was performed on TMA slides or whole sections using break apart probes for the DUSP22-IRF4 and TP63 loci and a dual-fusion probe for TBL1XR1/TP63 fusion [inv(3)(q26q28)]. Evaluation for rearrangements was performed without knowledge of the specific PTCL diagnosis, clinical course, or outcome.
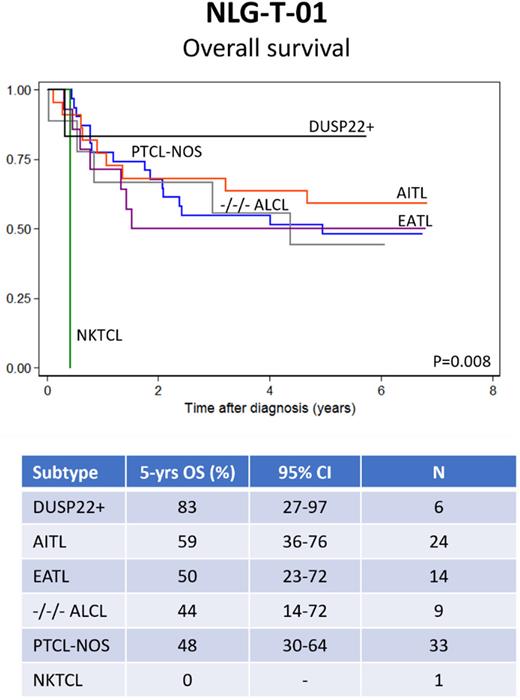
Results At a median FU of 10.7 years (range: 7-14 yrs), 71 of 160 (44,4%) intention-to-treat population patients were still alive and in remission. Causes of death for the 89 deceased patients were: lymphoma (76%), toxicity (10%), second malignancy (6%), other causes (8%). The 10-year OS, PFS and DSS for the whole ITTP were 41%, 38% and 51%, respectively (all CI95% within +/- 10% range). The multivariate analysis for long-term outcome-related parameters confirmed that female patients had a significantly better outcome than their male counterparts (10 yr OS: 68% vs 47%; HR 0.60 - CI95% 0.37-0.97; p=0.039). Elevated International Prognostic Index (IPI) parameters also retained their adverse prognostic impact, in particular age (pr year) (OS: HR 1.03 - CI95% 1.00-1.06; p=0.004 and PFS: HR 1.03 - CI95% 1.01-1.06; p=0.03) and poor performance score (PS ≥2) (OS: HR 1.71 - CI95% 1.08-2.72; p=0.023 and PFS: HR 1.84 - CI95% 1.18-2.86; p=0.009). DUSP22 analysis revealed presence of a classical DUSP22-IRF4 rearrangement in six patients. All of them achieved a complete remission (CR), four stayed in continuous CR for all the follow-up period, one experienced a late relapse approximately 5 years after end of 1st line treatment and responded to 2nd line treatment with a new sustained ongoing CR, and one died of sepsis after high-dose therapy while in CR. TP63 rearrangement was found in one patient, who responded to trial therapy and was in long-term CR at the time of follow-up analysis Fig.1 shows OS according to histological subtypes among the 87 patients analyzed for DUSP22 and TP63 rearrangements.
Conclusions Long-term follow up of the Nordic NLG-T-01 trial, confirmed a superior outcome for female patients and an adverse prognosis for elevated IPI parameters, in particular age (per year) and PS ≥ 2. A correlative biological analysis showed a favourable outcome for ALCL patients with DUSP22/IRF4 rearrranged tumours compared to other histological subtypes, including triple negative ALCL.
Disclosures
Holte:Incyte: Honoraria, Other: Advisory board; Gilead: Honoraria, Other: Advisory board; Novartis: Honoraria, Other: Advisory board; Takeda: Honoraria, Other: Advisory board; Nordic Nanovector: Honoraria, Other: Safety committee; Genmab: Honoraria, Other: Safety committee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal